Development of fMRI in awake behaving Nonhuman Primates
Non-invasive neuroimaging in human subjects often provides information that must be interpreted through models that are informed by prior invasive work in lower animals species, leaving an information gap due to differences in species, imaging methods, and anesthetic state. Neuroimaging in behaviing non-human primates helps bridge this gap. After our initial development of IRON fMRI in rodent models, we initiated a collaboration with the laboratory of Guy Orban and Wim Vanduffel to facilitate development of fMRI in awake NHP. Subsequently, we fully characterized BOLD and IRON fMRI in this model at our laboratory, extended methods to include event-related designs, and collaborated in a series of impactful neuroscience studies using this new method.
publications
Repeated fMRI Using Iron Oxide Contrast Agent in Awake, Behaving Macaques at 3 Tesla
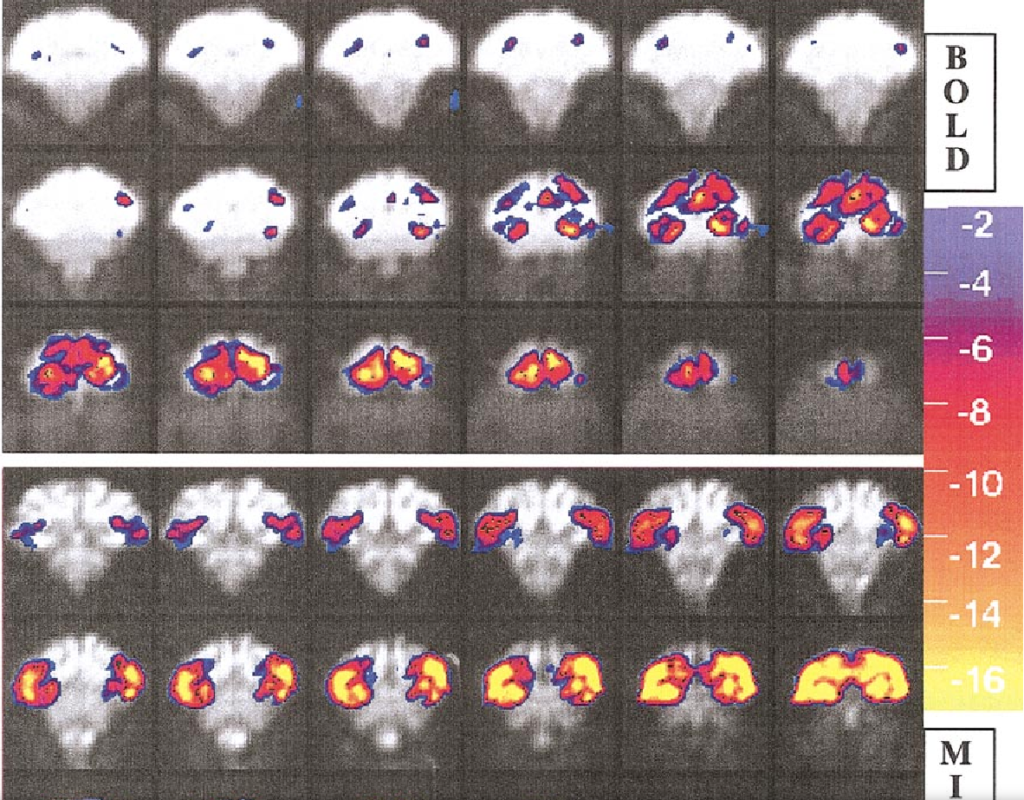
Iron oxide contrast agents have been employed extensively in anesthetized rodents to enhance fMRI sensitivity and to study the physiology of cerebral blood volume (CBV) in relation to blood oxygen level-dependent (BOLD) signal following neuronal activation. This study quantified the advantages of exogenous agent for repeated neuroimaging in awake, nonhuman primates using a clinical 3 Tesla scanner. A monocrystalline iron oxide nanoparticle (MION) solution was injected at iron doses of 8 to 10 mg/kg in two macaque monkeys. Adverse behavioral effects due to contrast agent were not observed in either monkey using cumulative doses in excess of 60 mg/kg. Relative to BOLD imaging at 3 Tesla, MION increased functional sensitivity by an average factor of 3 across the brain for a stimulus of long duration. Rapid stimulus presentation attenuated MION signal changes more than BOLD signal changes, due to the slower time constant of the blood volume response relative to BOLD signal. Overall, the contrast agent produced a dramatic improvement in functional brain imaging results in the awake, behaving primate at this field strength.
Characterization of event-related designs using BOLD and IRON fMRI. Neuroimage
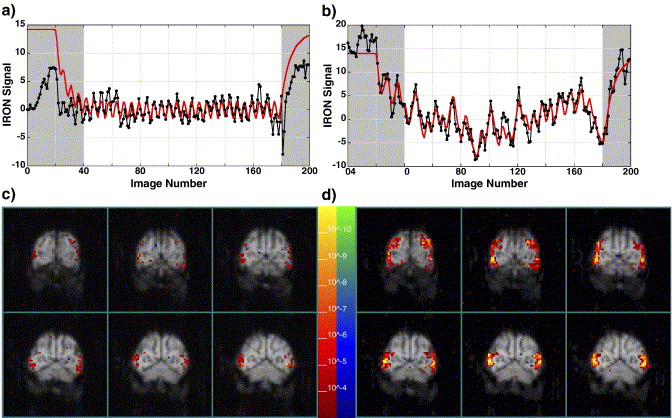
Despite many desirable characteristics, event-related (ER) stimulus designs for BOLD and IRON suffer from low detection power relative to block designs because the hemodynamic impulse response function (IRF) acts as a low-pass filter on neural activation to attenuate the size of differential responses to alternate stimuli. While the use of exogenous contrast agent (IRON technique) provides an alternative fMRI method in animal models to improve sensitivity and spatial localization, the inherently slower hemodynamic IRF causes IRON detection efficiency to decrease faster than BOLD efficiency as the interstimulus interval (ISI) is shortened. Using simulations based upon assumptions of stimulus–response linearity and experimental data obtained in awake, non-human primates, this study compared detection efficiencies for fixed, random and semi-random ISI distributions for BOLD and IRON techniques. A larger relative gain in detection efficiency at short ISI was obtained by randomized designs using IRON contrast relative to BOLD contrast due to the slower IRF of the IRON method. To quantify tradeoffs between detection efficiency and the predictability of stimulus presentation, the Shannon entropy was introduced as an objective measure of predictability. Small amounts of entropy can be traded for large gains in efficiency, particularly for the IRON method.
Faces and objects in macaque cerebral cortex
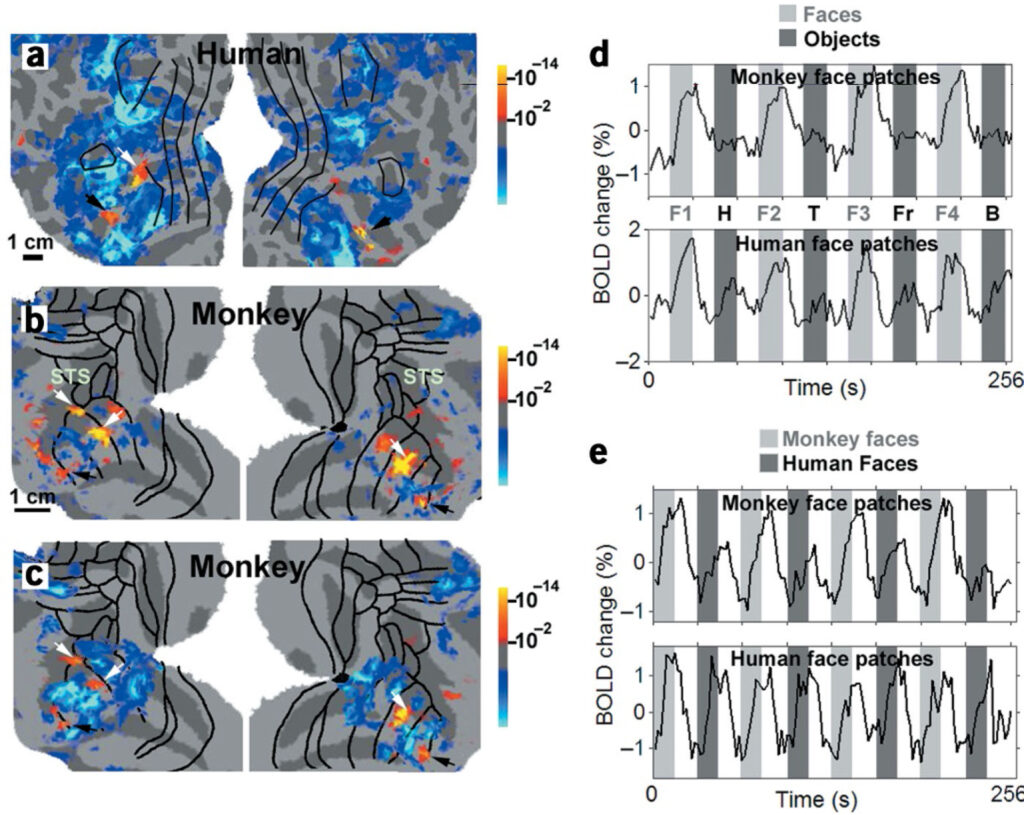
How are different object categories organized by the visual system? Current evidence indicates that monkeys and humans process object categories in fundamentally different ways. Functional magnetic resonance imaging (fMRI) studies suggest that humans have a ventral temporal face area, but such evidence is lacking in macaques. Instead, face-responsive neurons in macaques seem to be scattered throughout temporal cortex, with some relative concentration in the superior temporal sulcus (STS). Here, using fMRI in alert fixating macaque monkeys and humans, we found that macaques do have discrete face-selective patches, similar in relative size and number to face patches in humans. The face patches were embedded within a large swath of object-selective cortex extending from V4 to rostral TE. This large region responded better to pictures of intact objects compared to scrambled objects, with different object categories eliciting different patterns of activity, as in the human. Overall, our results suggest that humans and macaques share a similar brain architecture for visual object processing.