Contributions to fMRI physiology
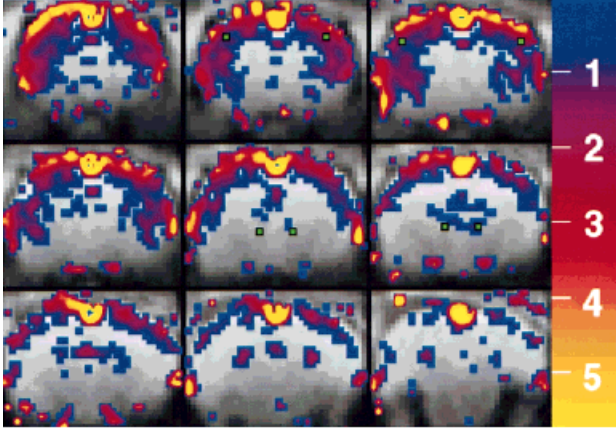
In our very first study using IRON fMRI (above), we noted that the temporal response of the CBV appeared to be significantly slower than BOLD signal, and we postulated that this explained the long-debated BOLD post-stimulus undershoot. Our hypothesis – a temporal mismatch between CBF and CBV – was confirmed in a subsequent study and published together with a Windkessel model of the response. This finding countered the prevailing notion that this feature of the response arose from post-stimulus metabolic activity required for restoration of oxygen stores, thereby providing an indirect indication of anaerobic metabolism. Although aspects of these phenomena remain controversial, the flow-volume temporal mismatch has been confirmed by numerous other investigators and incorporated into influential models of the functional response. The slow IRON temporal response affects routine experimental design and analyses, and it is evident in BOLD dynamics. Additionally, we performed the first “calibrated BOLD” measurements, which were reported first by Tim Davis and myself in separate 1997 abstracts. The “calibrated BOLD model” has become a standard way to extract the rate of oxygen utilization from BOLD signal, and my initial preclinical report was the first to incorporate changes in blood flow, volume, and BOLD signal into a complete measurement model for estimating oxygen utilization.
publications
Evidence of a Cerebrovascular Post-arteriole Windkessel with Delayed Compliance
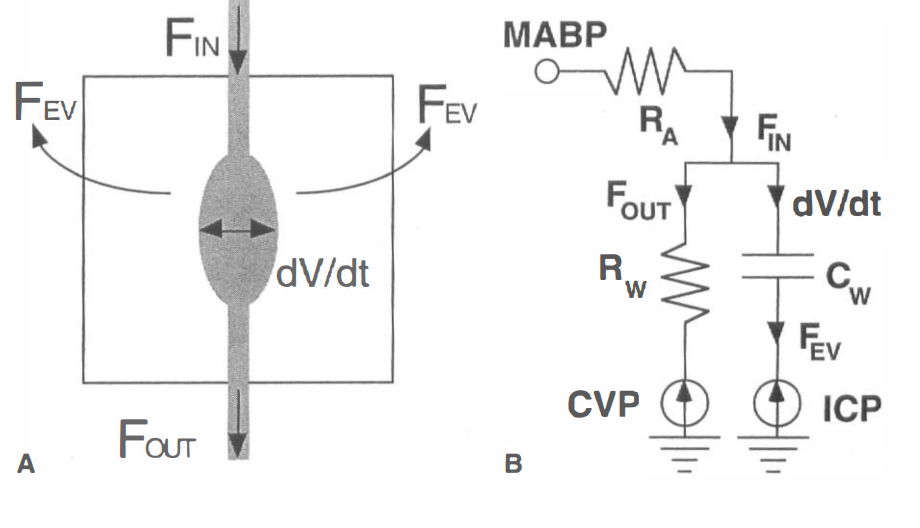
Evidence of a Cerebrovascular Post-arteriole Windkessel with Delayed Compliance
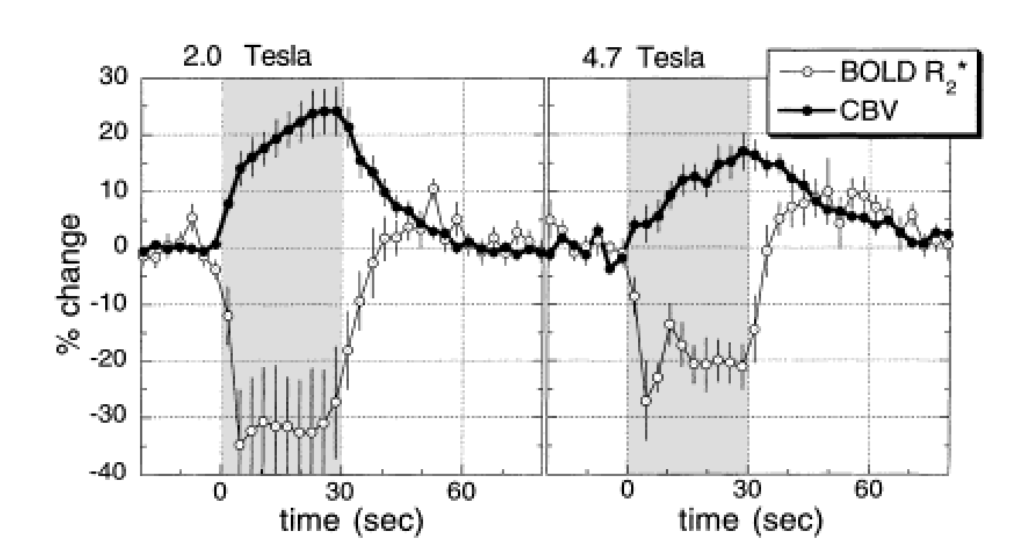
Evidence of a Cerebrovascular Post-arteriole Windkessel with Delayed Compliance
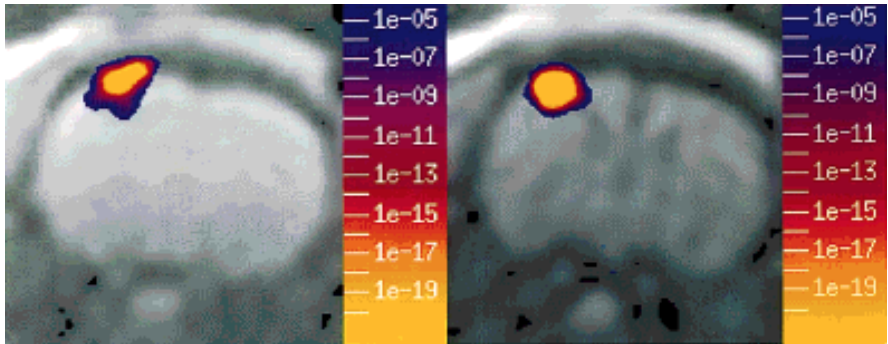
Evidence of a Cerebrovascular Post-arteriole Windkessel with Delayed Compliance