Development and application of pharmacological fMRI
Pharmacological fMRI, sometimes denoted “phMRI”, was one of our early target applications and a primary motivation for the development of IRON fMRI. The nature of these studies impacts imaging requirements and analysis strategies. Drugs generally induce cerebral responses that are long in duration and refractory in nature, placing limits on the ability to average multiple stimuli or to resample the baseline in order to monitor signal drift that is unrelated to the physiological response. Thus, optimizing the inherent detection power of the phMRI method is critical for these studies. Moreover, analyses need to be tailored to pick up variable regional temporal responses that can be inform underlying mechanisms. From an early cover article in Neuroimage reporting the acute effects of cocaine in anesthetized rats, to later fMRI studies of cocaine self-administration in macaques, our group has been at the forefront in developing and applying pharmacological fMRI in a wide variety of preclinical models. I recently reviewed phMRI acquisition and analysis methods in a special issue of Neuropharmacology.
publications
Cocaine activation discriminates dopaminergic projections by temporal response: an fMRI study in Rat
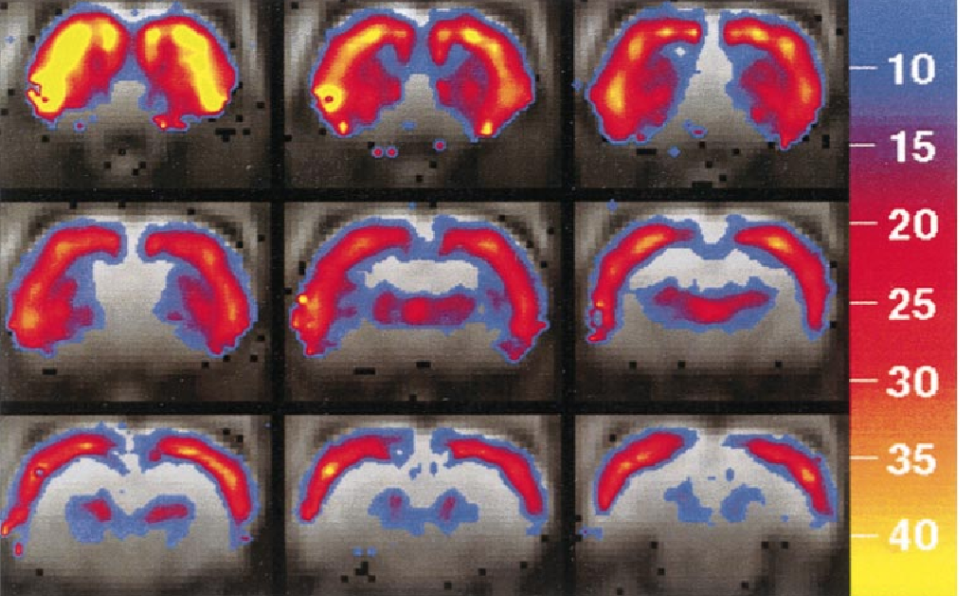
We applied a sensitive new functional magnetic resonance imaging technique to identify the pattern and determinants of cocaine-induced brain activation in drug-naive rats. At doses greater than 0.1 mg/kg iv, cocaine produced robust activation throughout cortex with the largest magnitude increase in frontal neocortex. Additionally, we detected selective activation within dopamine-innervated subcortical regions including dorsomedial and ventrolateral striatum, nucleus accumbens region, and dorsal thalamus. Although dose response was similar among activated regions, temporal response differentiated regions along distinct anatomical boundaries with basal ganglia and limbic cortical structures, reaching maximum activation later than frontal neocortex. Pharmacological specificity was demonstrated by blocking cocaine-induced activation with SCH-23390, a selective D1 antagonist. Our data demonstrate the utility of fMRI to identify spatiotemporal patterns of cocaine-induced brain activation and implicate D1 dopaminergic mechanisms in acute cocaine action.
fMRI of cocaine self-administration in macaques reveals functional inhibition of basal ganglia
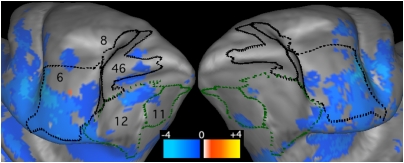
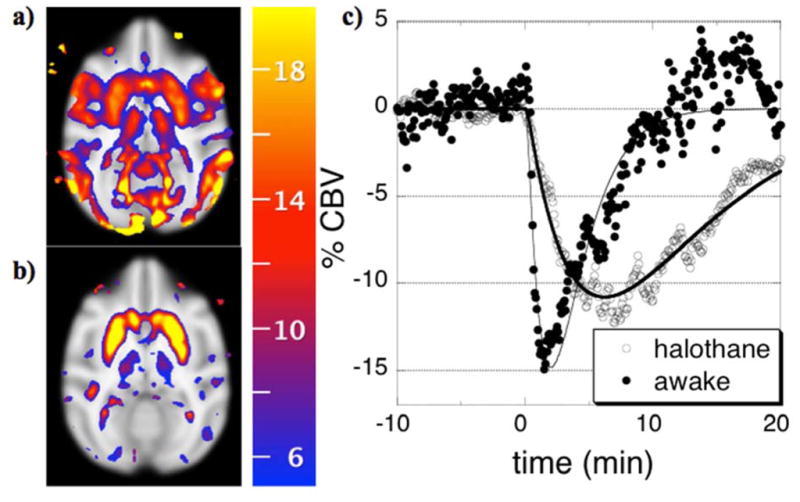
Disparities in cocaine-induced neurochemical and metabolic responses between human beings and rodents motivate the use of non-human primates (NHP) to model consequences of repeated cocaine exposure in human subjects. To characterize the functional response to cocaine infusion in NHP brain, we employed contrast-enhanced fMRI during both non-contingent injection of drug and self-administration of cocaine in the magnet. Cocaine robustly decreased cerebral blood volume (CBV) throughout basal ganglia and motor/pre-motor cortex and produced subtle functional inhibition of prefrontal cortex. No brain regions exhibited significant elevation of CBV in response to cocaine challenge. Theses effects in NHP brain are opposite in sign to the cocaine-induced fMRI response in rats, but consistent with previous measurements in NHP based on glucose metabolism. Because the striatal ratio of D2 to D1 receptors is larger in human beings and NHP than rats, we hypothesize that the inhibitory effects of D2 receptor binding dominate the functional response in primates, whereas excitatory D1 receptor stimulation predominates in the rat. If the NHP accurately models the human response to cocaine, downregulation of D2 receptors in human cocaine-abusing populations can be expected to blunt cocaine-induced functional responses, contributing to the weak and variable fMRI responses reported in human basal ganglia following cocaine infusion.
Remifentanil administration reveals biphasic phMRI temporal responses in rat consistent with dynamic receptor regulation
Many pharmacological stimuli influence multiple neurotransmitter systems in the brain, and the dynamics of the functional brain response can vary regionally. In this study, the temporal response of cerebral blood volume (CBV) was employed to spatially segment cerebral effects due to infusion of a potent mu-opioid receptor agonist. Repeated intravenous injection of 10 ug/kg remifentanil in rats caused reproducible regional positive, negative, and biphasic changes in CBV. Three temporal processes were identified in the cerebral response and analyzed within the framework of the general linear model. Firstly, a slow component identified CBV changes that were almost exclusively negative, and the spatial distribution was similar to the inhibition produced by morphine (200 ug/kg). The largest CBV reductions occurred in caudate, accumbens, ventral hippocampus, cingulate, and piriform cortex. Secondly, a more rapid temporal component corresponded primarily with a regional distribution of positive changes in CBV consistent with GABAergic inhibition of hippocampal interneurons and associated projections. Thirdly, a response with the dynamics of mean arterial blood pressure correlated positively with CBV changes in hypothalamus, consistent with a central mechanism for control of blood pressure. We propose that the dominant source of the temporal variance in signal is dynamic modulation of drug targets by receptor endocytosis, an established effect in vitro. These results suggest that the temporal response of fMRI signal reflects underlying neurobiological processes, so that temporal decomposition strategies may aid interpretation of pharmacological mechanisms by identifying interconnected regions or those associated with common neural targets and processes.
Data Collection and Analysis Strategies for phMRI
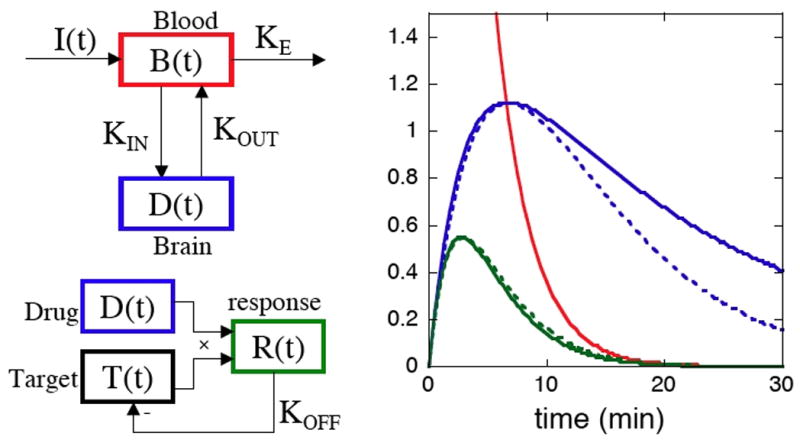
Although functional MRI traditionally has been applied mainly to study changes in task-induced brain function, evolving acquisition methodologies and improved knowledge of signal mechanisms have increased the utility of this method for studying responses to pharmacological stimuli, a technique often dubbed “phMRI”. The proliferation of higher magnetic field strengths and the use of exogenous contrast agent have boosted detection power, a critical factor for successful phMRI due to the restricted ability to average multiple stimuli within subjects. Receptor-based models of neurovascular coupling, including explicit pharmacological models incorporating receptor densities and affinities and data-driven models that incorporate weak biophysical constraints, have demonstrated compelling descriptions of phMRI signal induced by dopaminergic stimuli. This report describes phMRI acquisition and analysis methodologies, with an emphasis on data-driven analyses. As an example application, statistically efficient data-driven regressors were used to describe the biphasic response to the mu-opioid agonist remifentanil, and antagonism using dopaminergic and GABAergic ligands revealed modulation of the mesolimbic pathway. Results illustrate the power of phMRI as well as our incomplete understanding of mechanisms underlying the signal. Future directions are discussed for phMRI acquisitions in human studies, for evolving analysis methodologies, and for interpretative studies using the new generation of simultaneous PET/MRI scanners