occupancy/function measurements and models using simultaneous PET/fMRI
The recent generation of PET/MR scanners has provided opportunities for investigations that previously would have been arduous. We have explored classic paradigms of pharmacology that only now can be accomplished in vivo by comparing PET measurements of receptor occupancy with induced fMRI responses. Using a dopamine D2/D3 receptor antagonist, we demonstrated correlations between receptor occupancy and induced function in the domains of space, time, and dose, consistent with a classical receptor model in which binding drives function. Conversely, using a high affinity D2/D2 agonist that is known to induce receptor internalization, we demonstrated a profound temporal mismatch between occupancy and function, and our biophysical model of the data produced an estimate for the rate of desensitization and internalization that is consistent with in vitro data. Using these principles, we developed a multi-receptor model of dopamine-driven fMRI signal in basal ganglia, and we showed that this simple model can reconcile many literature observations that previously were viewed as discrepant, including changes in the sign of the fMRI and metabolic responses to some drugs across species, and changes in the sign of the response versus the level of induced dopamine. These studies clarify the functional consequences of dopaminergic stimulation and provide a biochemical foundation for understanding fMRI signal using pharmacological stimuli, and moreover define a general paradigm for future studies of other neuroreceptor systems. Furthermore, we have investigated effects of flow on radiotracers, and refined reference-region models for quantifying changes in receptor occupancy.
publications
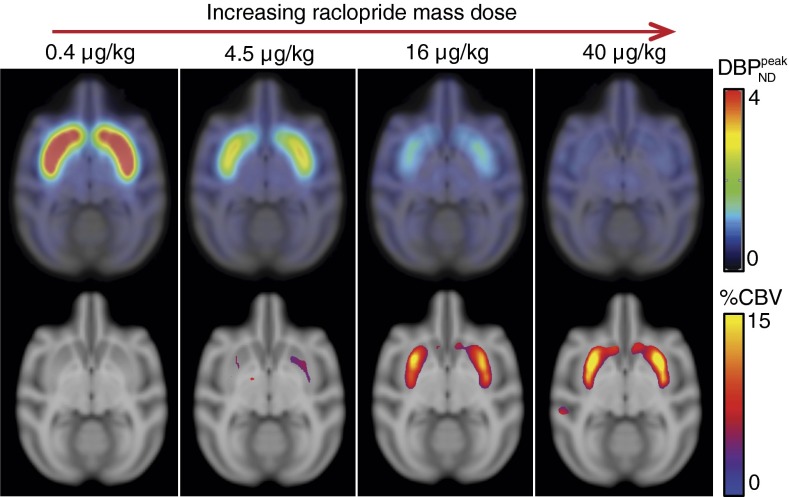
A receptor-based model for dopamine-induced fMRI signal
This report describes a multi-receptor physiological model of the fMRI temporal response and signal magnitude evoked by drugs that elevate synaptic dopamine in basal ganglia. The model is formulated as a summation of dopamine’s effects at D1-like and D2-like receptor families, which produce functional excitation and inhibition, respectively, as measured by molecular indicators like adenylate cyclase or neuroimaging techniques like fMRI. Functional effects within the model are described in terms of relative changes in receptor occupancies scaled by receptor densities and neuro-vascular coupling constants. Using literature parameters, the model reconciles many discrepant observations and interpretations of pre-clinical data. Additionally, we present data showing that amphetamine stimulation produces fMRI inhibition at low doses and a biphasic response at higher doses in the basal ganglia of non-human primates (NHP), in agreement with model predictions based upon the respective levels of evoked dopamine. Because information about dopamine release is required to inform the fMRI model, we simultaneously acquired PET 11C-raclopride data in several studies to evaluate the relationship between raclopride displacement and assumptions about dopamine release. At high levels of dopamine release, results suggest that refinements of the model will be required to consistently describe the PET and fMRI data. Overall, the remarkable success of the model in describing a wide range of preclinical fMRI data indicate that this approach will be useful for guiding the design and analysis of basic science and clinical investigations and for interpreting the functional consequences of dopaminergic stimulation in normal subjects and in populations with dopaminergic neuroadaptations.
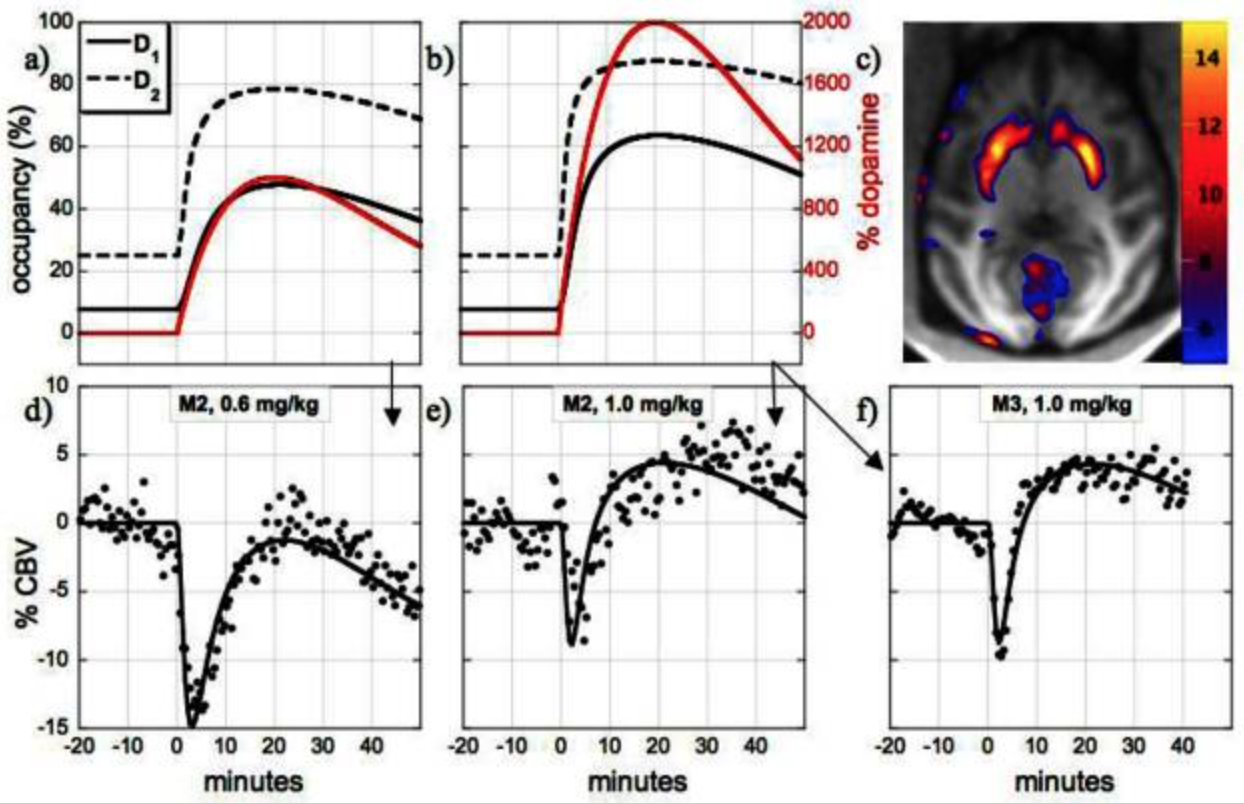
Neurovascular coupling to D2/D3 dopamine receptor occupancy using simultaneous PET/functional MRI
This study employed simultaneous neuroimaging with positron emission tomography (PET) and functional magnetic resonance imaging (fMRI) to demonstrate the relationship between changes in receptor occupancy measured by PET and changes in brain activity inferred by fMRI. By administering the D2/D3 dopamine receptor antagonist [11C]raclopride at varying specific activities to anesthetized nonhuman primates, we mapped associations between changes in receptor occupancy and hemodynamics [cerebral blood volume (CBV)] in the domains of space, time, and dose. Mass doses of raclopride above tracer levels caused increases in CBV and reductions in binding potential that were localized to the dopamine-rich striatum. Moreover, similar temporal profiles were observed for specific binding estimates and changes in CBV. Injection of graded raclopride mass doses revealed a monotonic coupling between neurovascular responses and receptor occupancies. The distinct CBV magnitudes between putamen and caudate at matched occupancies approximately matched literature differences in basal dopamine levels, suggesting that the relative fMRI measurements reflect basal D2/D3 dopamine receptor occupancy. These results can provide a basis for models that relate dopaminergic occupancies to hemodynamic changes in the basal ganglia. Overall, these data demonstrate the utility of simultaneous PET/fMRI for investigations of neurovascular coupling that correlate neurochemistry with hemodynamic changes in vivo for any receptor system with an available PET tracer.
Imaging Agonist-Induced D2/D3 Receptor Desensitization and Internalization In Vivo with PET/fMRI
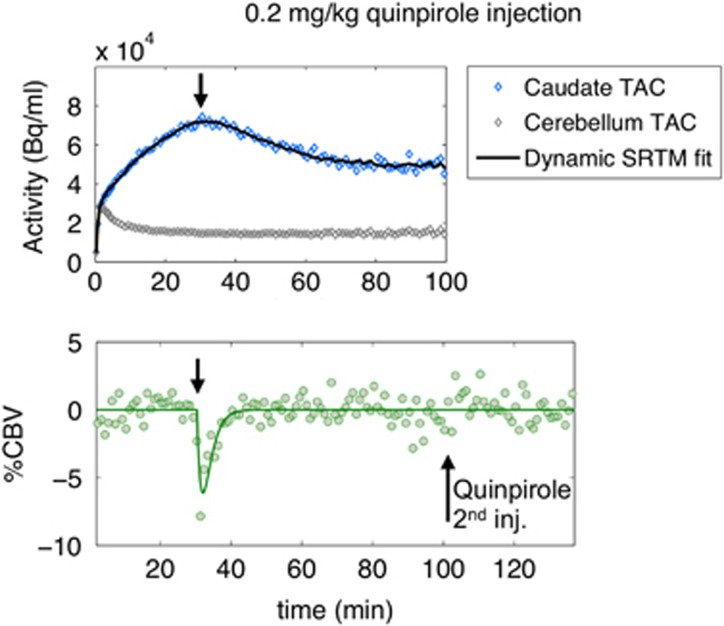
This study investigated the dynamics of dopamine receptor desensitization and internalization, thereby proposing a new technique for non-invasive, in vivo measurements of receptor adaptations. The D2/D3 agonist quinpirole, which induces receptor internalization in vitro, was administered at graded doses in non-human primates while imaging with simultaneous positron emission tomography (PET) and functional magnetic resonance imaging (fMRI). A pronounced temporal divergence between receptor occupancy and fMRI signal was observed: occupancy remained elevated while fMRI responded transiently. Analogous experiments with an antagonist (prochlorperazine) and a lower-affinity agonist (ropinirole) exhibited reduced temporal dissociation between occupancy and function, consistent with a mechanism of desensitization and internalization that depends upon drug efficacy and affinity. We postulated a model that incorporates internalization into a neurovascular-coupling relationship. This model yielded in vivo desensitization/internalization rates (0.2/min for quinpirole) consistent with published in vitro measurements. Overall, these results suggest that simultaneous PET/fMRI enables characterization of dynamic neuroreceptor adaptations in vivo, and may offer a first non-invasive method for assessing receptor desensitization and internalization.
A regularized full reference tissue model for PET neuroreceptor mapping
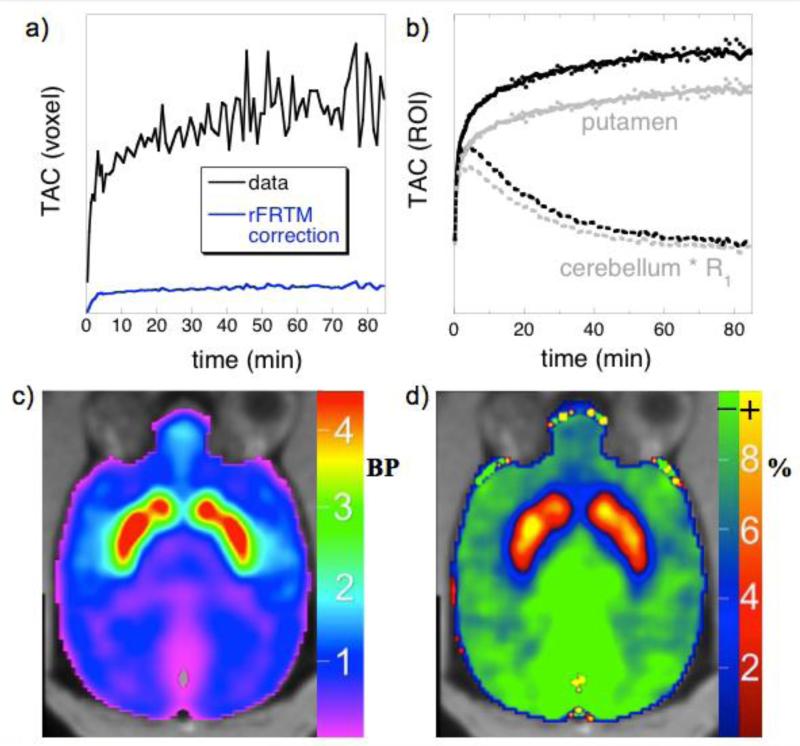
The full reference tissue model (FRTM) is a PET analysis framework that includes both free and specifically bound compartments within tissues, together with rate constants defining association and dissociation from the specifically bound compartment. The simplified reference tissue model (SRTM) assumes instantaneous exchange between tissue compartments, and this “1-tissue” approximation reduces the number of parameters and enables more robust mapping of non-displaceable binding potentials. Simulations based upon FRTM have shown that SRTM exhibits biases that are spatially dependent, because biases depend upon binding potentials. In this work, we describe a regularized model (rFRTM) that employs a global estimate of the dissociation rate constant from the specifically bound compartment (k4). The model provides an internal calibration for optimizing k4 through the reference-region outflow rate k2′, a model parameter that should be a global constant but varies regionally in SRTM. Estimates of k4 by rFRTM are presented for four PET radioligands. We show that SRTM introduces bias in parameter estimates by assuming an infinite value for k4, and that rFRTM ameliorates bias with an appropriate choice of k4. Theoretical considerations and simulations demonstrate that rFRTM reduces bias in non-displaceable binding potentials. A two-parameter reduction of the model (rFRTM2) provides robust mapping at a voxel-wise level. With a structure similar to SRTM, the model is easily implemented and can be applied as a PET reference region analysis that reduces parameter bias without substantially altering parameter variance.